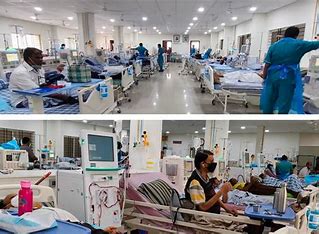
The world’s largest vaccine production company, Serum Institute of India (SII), has written a letter to the PMO on reforms in the existing drug regulatory system, including allowing companies manufacturing and stockpiling of non-Covid vaccines while undergoing clinical trials. In the letter Prakash Kumar Singh, who is the Director of Government and Regulatory Affairs at the Pune-based SII referred to the Union Health Ministry’s 18 May 2020 gazette notification, saying it allowed manufacturing and stockpiling of Covid-19 vaccine under clinical trial for marketing authorisation sale or distribution.
In the letter, Prakash Kumar Sing wrote, “Because of this rule, it became possible for us to manufacture and stockpile the Covid-19 vaccine during clinical trials and we could make the vaccine available in such a span of time to protect millions of lives.”