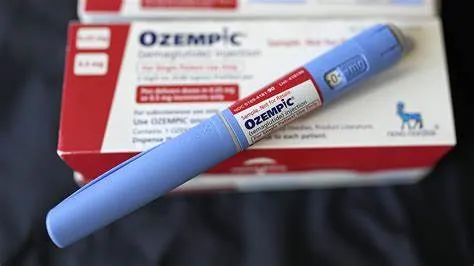
The U.S. Food and Drug Administration (FDA) has ordered compounding pharmacies to cease manufacturing less expensive versions of popular weight-loss drugs like Ozempic and Zepbound. This move follows federal officials’ determination that there is no longer a shortage of glucagon-like peptide-1 (GLP-1) drugs. The ban affects medications containing active ingredients trizepatide and semaglutide, with the prohibition on trizepatide-based drugs taking effect on March 19 and the semaglutide ban scheduled for April 22.
Compounding pharmacies have provided an affordable alternative for patients unable to access brand-name drugs, which are often not covered by insurance. The FDA’s decision to enforce the ban has ignited concerns about affordability and access for patients who have relied on these compounded medications to manage weight effectively.
What Are GLP-1 Drugs and Why Are They Popular?
GLP-1 receptor agonists, including Ozempic, Zepbound, Wegovy, and Mounjaro, are medications originally designed to treat type 2 diabetes but have been widely used off-label for weight loss. These drugs work by mimicking the GLP-1 hormone, which regulates blood sugar levels and suppresses appetite, making patients feel less hungry.
Due to their effectiveness, these medications have become an integral part of weight management strategies for many individuals. Alongside a prescribed diet and exercise regimen, GLP-1 drugs help patients achieve sustainable weight loss, offering a more medically backed alternative to diet pills and unregulated supplements.
Dr. Mir Ali, a general and bariatric surgeon, emphasizes the importance of these medications in weight management. “These drugs target the root causes of weight gain, improving the likelihood of long-term success,” Dr. Ali explains. He also highlights the shift in medical perspectives, with obesity increasingly viewed as a chronic disease requiring targeted treatment.
The FDA’s Decision and Its Impact on Compounding Pharmacies
Compounded drugs are custom-made medications prepared by licensed pharmacies to meet individual patient needs when FDA-approved versions are unavailable or unsuitable. These drugs are not FDA-approved, meaning the agency does not verify their safety, efficacy, or quality. Nevertheless, they can be legally sold under specific conditions.
The FDA’s updated guidance follows a federal judge’s ruling against the Outsourcing Facilities Association (OFA), which represented compounding pharmacies producing copycat trizepatide medications. A similar lawsuit concerning semaglutide compounds is under consideration.
The ban on compounded versions of weight-loss drugs coincides with the FDA’s announcement that the shortage of GLP-1 drugs had ended as of December 2024. The FDA allowed a transition period for compounding pharmacies to halt production, reinforcing Eli Lilly and Novo Nordisk’s exclusive rights to their respective products.
Why Are Patients Concerned?
Many patients who have successfully managed their weight with compounded GLP-1 drugs now face a difficult choice: pay significantly more for brand-name versions or stop the medication and risk regaining weight. Patients who rely on compounded drugs often find brand-name options prohibitively expensive and inadequately covered by insurance.
For instance, Bailey Fields, a 27-year-old with polycystic ovarian syndrome (PCOS), pays $199 per month for her semaglutide medication through Hims & Hers, a telehealth provider. Previously, her Mounjaro treatment cost $675 per month. Bailey’s insurance would only cover weight-loss drugs if she developed type 2 diabetes, forcing her to choose between her health and finances. She expresses uncertainty about the future: “I don’t know what will happen if I stop taking it.”
Similarly, Todd Kennedy, 45, from Tennessee, pays $165 per month for compounded semaglutide. The brand-name alternative would cost him approximately $1,500 per month—an unaffordable increase. Kennedy criticizes the FDA’s decision as “short-sighted,” emphasizing the drug’s importance in his weight management journey.
Dr. Ali warns that this decision could drive patients toward less regulated markets, risking exposure to counterfeit or unsafe medications. “People might not get what they pay for,” he cautions.
The Role of Telehealth Providers
Telehealth companies like Eden, Mochi, Ro, and Hims & Hers have been key players in providing compounded GLP-1 drugs since December 2022. The increased demand for weight-loss drugs led to supply shortages, prompting these providers to step in and offer alternatives under regulatory allowances.
Ro and Hims & Hers reaffirmed their commitment to helping patients. Ro stated, “We will continue to work to ensure that our patients can access the best treatments for their individual needs, following FDA rules.” Hims & Hers stressed the importance of affordable and consistent access to GLP-1 drugs, given that obesity affects over 40% of Americans.
Despite these reassurances, Hims & Hers reported an ongoing shortage of GLP-1 drugs in the U.S., raising doubts about the adequacy of future drug supplies.
Supply and Pricing Concerns
A pressing issue is whether Eli Lilly and Novo Nordisk can meet demand for GLP-1 medications following the ban on compounded versions. The absence of compounding pharmacies could strain the supply chain, potentially leading to delays or shortages.
Additionally, the FDA’s decision raises fears of price hikes. However, Dr. Ali speculates that brand-name manufacturers may maintain prices due to public and regulatory pressure to keep weight-loss medications affordable. Still, price concerns remain significant for uninsured patients or those whose insurance does not cover GLP-1 drugs.
Alternatives to GLP-1 Drugs
For some patients, alternatives like gastric bypass surgery may be considered. This surgical option offers long-term weight loss solutions but carries its own risks and costs. Dr. Ali notes that while surgery can be effective, it’s not suitable for everyone and should be considered after evaluating all other options.
Lifestyle interventions—diet and exercise—remain crucial components of weight management. Patients are advised to consult healthcare professionals before making any changes to their medication or treatment plan.
Broader Implications for Weight Management
The FDA’s decision to ban compounded GLP-1 drugs underscores broader issues in the healthcare system regarding access to affordable medication. Obesity is a global health challenge, and weight-loss drugs are vital in managing this epidemic. Limiting access to effective treatments could reverse progress in obesity management.
The decision also highlights the tension between drug affordability and patent protections. Pharmaceutical companies argue that patents incentivize innovation, while critics contend that high prices exclude patients who need medications the most.
The controversy over compounded GLP-1 drugs reflects ongoing debates in the U.S. healthcare system about balancing drug innovation, affordability, and access. The outcome of this decision could set precedents for other high-demand medications in the future.
The FDA’s ban on compounded versions of weight-loss medications like Ozempic and Zepbound reflects an effort to protect drug patents and ensure quality control in drug manufacturing. However, this move poses significant challenges for patients who rely on more affordable compounded versions to manage their weight.
Patients and healthcare providers are navigating a complex landscape of drug availability, affordability, and insurance coverage. As pharmaceutical companies ramp up production to meet demand, stakeholders hope for a balance between innovation, access, and cost-effectiveness in obesity management.