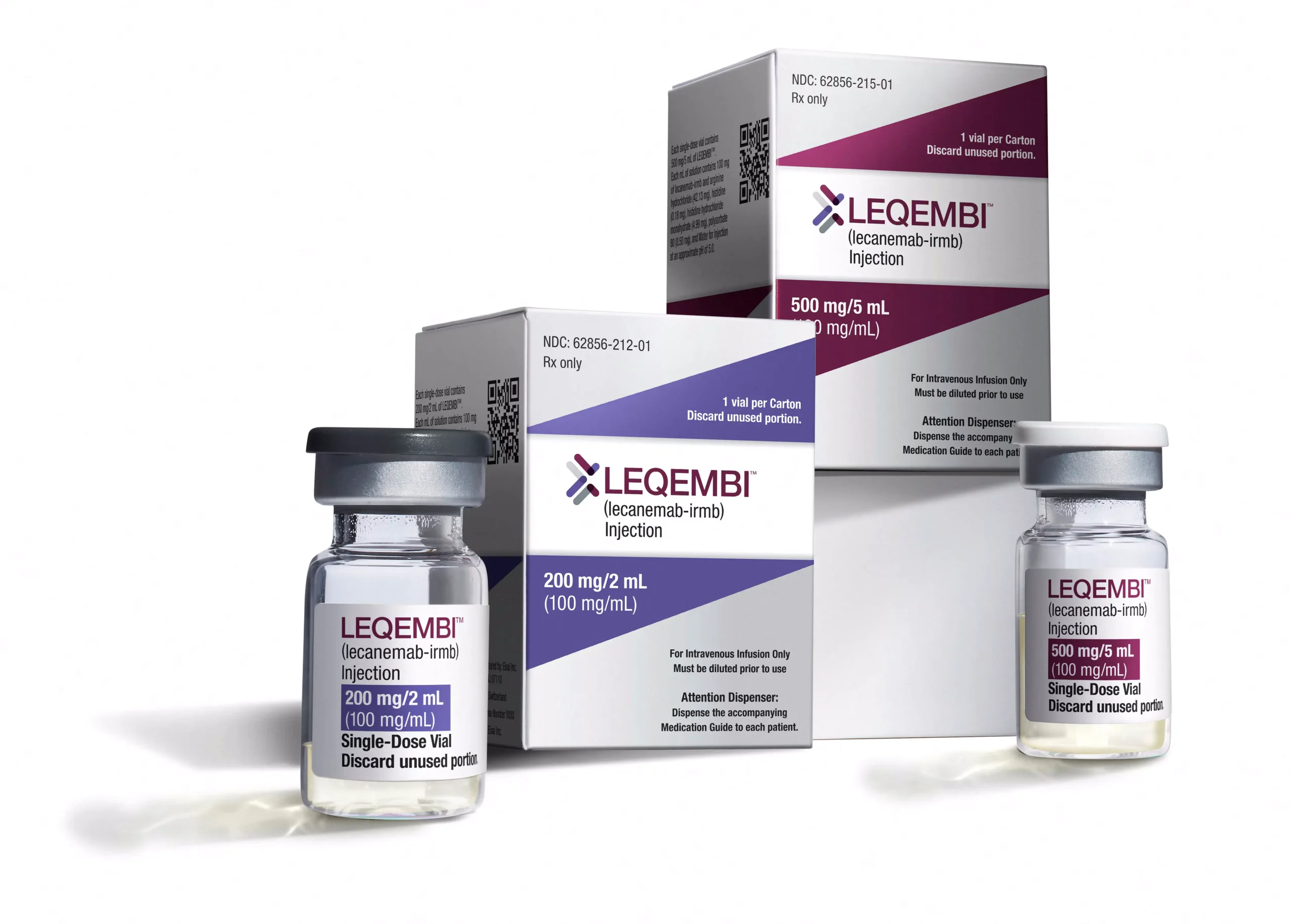
The U.S. Food and Drug Administration (FDA) has approved monthly maintenance dosing for Leqembi, an Alzheimer’s drug developed by Japan’s Eisai in partnership with Biogen. This approval marks a significant milestone in Alzheimer’s treatment, providing a more convenient dosing schedule for patients. Leqembi, which received standard approval from the FDA in 2023, is designed to treat Alzheimer’s by targeting and clearing amyloid beta deposits in the brain, a protein that is thought to contribute to the progression of the disease.
The Approval of Monthly Maintenance Dosing
This latest approval allows patients to switch from the original bi-weekly intravenous infusions of the drug to a more manageable monthly dosing schedule, after having completed 18 months of the bi-weekly regimen. While patients can continue with the bi-weekly dosing, the monthly maintenance dose offers a more patient-friendly option. The bi-weekly administration process, which occurs in an infusion center, typically lasts about an hour, requiring significant time commitment and regular MRI scans for monitoring. The new monthly dosing option is expected to be more convenient for patients, potentially improving adherence and overall quality of life.
The FDA’s decision came after the companies presented modeling simulations based on clinical trial data, which predicted that the monthly dosing would continue to maintain the therapeutic benefits of the original two-week schedule. This approval is particularly important given that Alzheimer’s treatment options have historically been limited, and current therapies mainly address symptoms rather than halting or reversing the disease’s progression. By offering a maintenance schedule that better fits into the lives of patients, Leqembi could become a more viable treatment option for many.
The Science Behind Leqembi
Leqembi works by targeting and removing amyloid beta plaques from the brain, which are associated with the cognitive decline seen in Alzheimer’s disease. These plaques are thought to interfere with communication between brain cells, leading to memory loss, confusion, and other cognitive impairments characteristic of Alzheimer’s. The removal of amyloid plaques has been shown to slow the progression of the disease in clinical trials, although it does not cure Alzheimer’s.
The drug is administered intravenously in an infusion center, where patients receive the medication under the supervision of healthcare professionals. Though the infusion process is relatively quick, it requires patients to schedule regular visits, making it a time-consuming treatment option. While the bi-weekly regimen was effective in clinical trials, the new monthly maintenance dose promises to offer patients more flexibility.
Growing Competition in Alzheimer’s Treatment
Leqembi faces competition in the Alzheimer’s treatment space from a rival drug called Kisunla, developed by Eli Lilly. Kisunla was approved by the FDA in July and is also used to treat Alzheimer’s by targeting amyloid beta plaques. What sets Kisunla apart, however, is its administration schedule—it is given once a month, rather than bi-weekly, making it an attractive option for patients seeking a less frequent dosing schedule. The treatment for Kisunla stops once brain scans no longer show amyloid plaques, further differentiating it from Leqembi’s maintenance approach.
While both Leqembi and Kisunla offer hope to Alzheimer’s patients, they also come with safety concerns, particularly regarding the potential for brain swelling and bleeds. Both medications carry warnings about these risks, which require careful monitoring with scans during treatment. The requirement for regular brain scans is one of the significant barriers to broader use of these medications, as it adds to the overall burden of treatment. Nevertheless, the approval of monthly maintenance dosing for Leqembi is likely to mitigate some of the concerns related to the frequency of treatments and the burden on patients.
The Slow Growth of Leqembi
Despite its promising clinical results, the growth of Leqembi has been slower than expected. One of the primary reasons for this is the complexity and time-consuming nature of its administration. With bi-weekly intravenous infusions, regular MRIs, and other screenings required, many patients may have been discouraged by the frequency of visits and the logistical burden that comes with it.
However, the FDA’s approval of monthly maintenance dosing could significantly increase the drug’s appeal to a broader patient population. By making the treatment more convenient, it’s possible that the number of patients willing to start and adhere to the therapy could rise. Additionally, the monthly dosing option may encourage physicians to prescribe the drug more widely, potentially resulting in higher adoption rates.
Safety Concerns and Monitoring
Both Leqembi and Kisunla have safety warnings regarding the risks of brain swelling and bleeding. These potential side effects are serious and require close monitoring through regular brain scans. Brain swelling, known as ARIA (Amyloid-Related Imaging Abnormalities), can lead to significant complications, including stroke-like symptoms such as headaches, dizziness, and confusion. The risk of these side effects has raised concerns about the safety of these drugs, even though the benefits of amyloid plaque clearance are clear in clinical trials.
For Leqembi, Eisai and Biogen have emphasized the importance of ongoing monitoring, particularly in the initial stages of treatment. The drugs are typically given to patients with moderate Alzheimer’s, a stage at which the brain is already undergoing significant changes. As such, the safety of the treatment must be carefully weighed against its potential benefits.
The Role of Leqembi in Alzheimer’s Treatment
Alzheimer’s disease is a complex and devastating condition that currently affects millions of individuals worldwide, with that number expected to rise dramatically as populations age. While there are a few other Alzheimer’s treatments available, they primarily focus on alleviating symptoms rather than targeting the underlying disease processes. Leqembi’s ability to clear amyloid plaques represents a significant advancement in the treatment of the disease, offering hope that future therapies could modify the course of Alzheimer’s or even halt its progression entirely.
Despite its promising mechanism of action, Leqembi is still not a cure for Alzheimer’s. Patients and healthcare providers must continue to manage the disease with a combination of drug therapies, lifestyle interventions, and cognitive support. However, the progress made by Leqembi and similar drugs opens the door for future treatments that may provide even more substantial benefits to patients.
The approval of monthly maintenance dosing for Leqembi marks a significant milestone in the ongoing fight against Alzheimer’s disease. By making the treatment more accessible and less burdensome, the monthly dosing option has the potential to improve adherence and patient outcomes. Although there are safety concerns that must be carefully monitored, Leqembi’s ability to slow cognitive decline in Alzheimer’s patients offers hope for a population facing a devastating condition with few treatment options.
With growing competition from other drugs like Kisunla, Leqembi’s success will depend on its ability to balance efficacy, convenience, and safety. As research into Alzheimer’s continues and more treatments become available, it is possible that the future will bring more effective therapies, ultimately providing patients and their families with better management options for this debilitating disease. For now, Leqembi stands as a beacon of progress in Alzheimer’s treatment, and the approval of its monthly maintenance dosing is a significant step forward in making the drug more widely accessible.