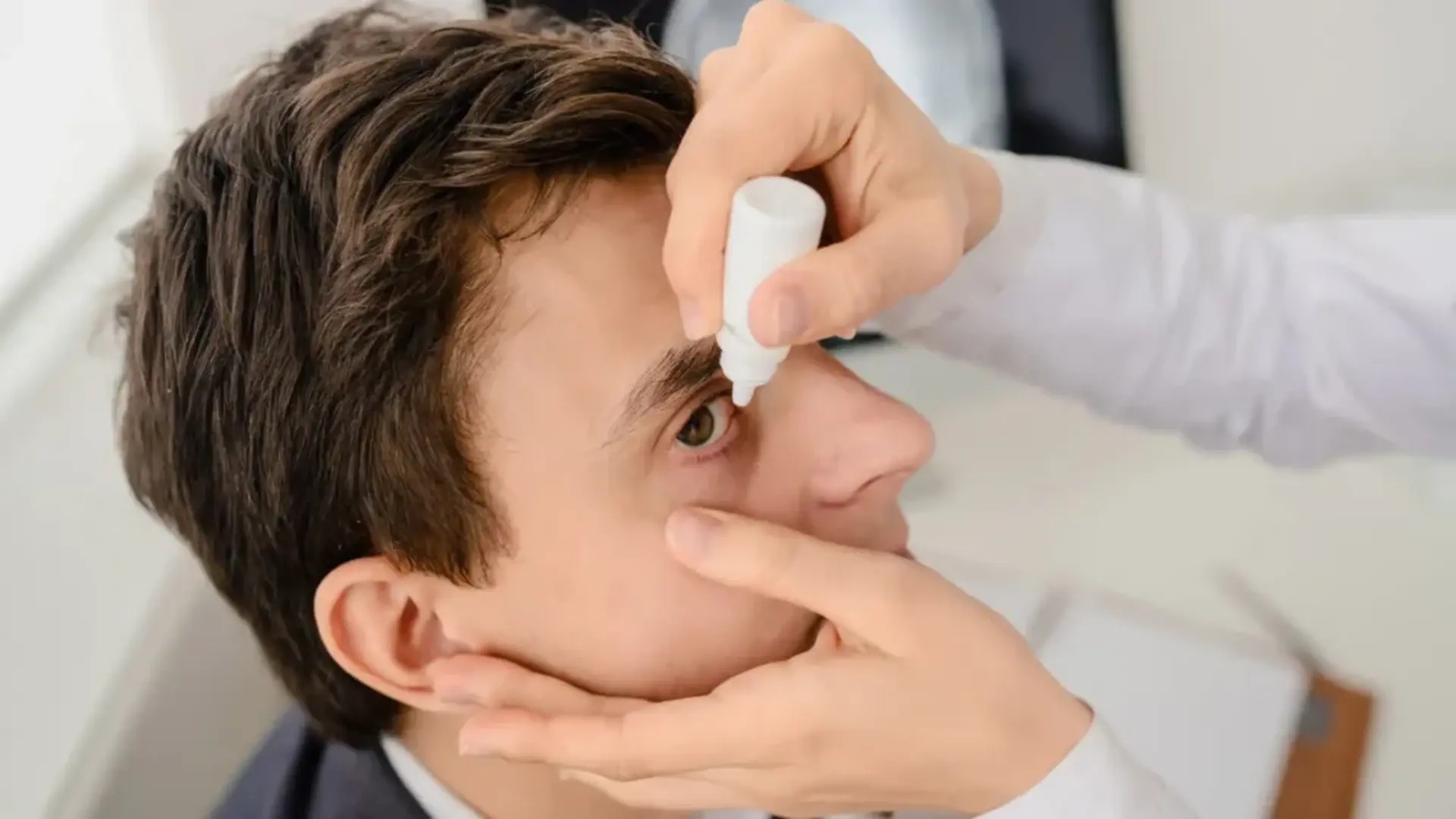
Concerns about the misuse of eye drops designed to replace reading glasses have halted their release, following the suspension of marketing approvals by the Drug Controller General of India (DCGI).
Presbyopia, a common age-related vision issue that makes focusing on nearby objects difficult, was the target condition for these eye drops. The manufacturer claimed that a single drop would start working within 15 minutes, with effects lasting up to six hours. An additional drop, administered within three to six hours, was said to extend the effect. Priced at Rs 340, the eye drops were scheduled to hit pharmacies in October.
However, on September 10, the DCGI issued an order suspending the product’s approval, citing concerns over exaggerated claims made by the company. The Central Drugs Standard Control Organization (CDSCO), under the Union Ministry of Health and Family Welfare, took swift action due to the risk of public misuse, particularly after the product was promoted as an over-the-counter drug despite being prescription-based.
Also read: Entod Pharmaceuticals Faces Allegations of “Unethical and False Presentation” Over PresVu Eye Drops
Why Were the Eye Drops Suspended?
According to the DCGI’s order, the eye drops—Pilocarpine Hydrochloride Ophthalmic Solution USP 1.25%—were approved for treating presbyopia in adults. However, the manufacturer, Entod Pharmaceuticals, made unsubstantiated claims that the drops could reduce the need for reading glasses. Regulatory authorities were dissatisfied with the company’s responses to these claims.
The Key Claims Under Scrutiny:
First Eye Drops in India to Reduce the Need for Reading Glasses
The manufacturer asserted that no other similar product existed in India. The DCGI disagreed, clarifying that the eye drops had not been approved to reduce reliance on glasses.
Non-Invasive Solution to Enhance Near Vision Without Glasses
The company cited clinical trials where participants did not wear glasses. The regulator, however, emphasized that the approval was only for treating presbyopia, not for enhancing vision without glasses.Vision Improvement in 15 Minutes
The claim that the drops provide a near-immediate alternative to reading glasses also did not hold up. The DCGI stated that the eye drops were not approved for such a rapid effect.Regulatory Concerns and Expert Opinions
The regulatory agency concluded that Entod Pharmaceuticals violated drug laws by promoting claims without prior approval from the central licensing authority. As a result, the product has been suspended under Rule 84 of the New Drugs and Clinical Trials Rules, 2019.Multiple eye care experts have raised concerns about the long-term safety and efficacy of such products. While eye drops may offer temporary relief, replacing spectacles entirely with reusable drops is not advisable. According to ophthalmologists, these drops could only serve as a short-term solution rather than a sustainable alternative to glasses.
What’s Next?
The case highlights the importance of stringent drug regulations, especially for products claiming to offer easy alternatives to medical devices like glasses. Until further review and approval, the future of these eye drops remains uncertain.