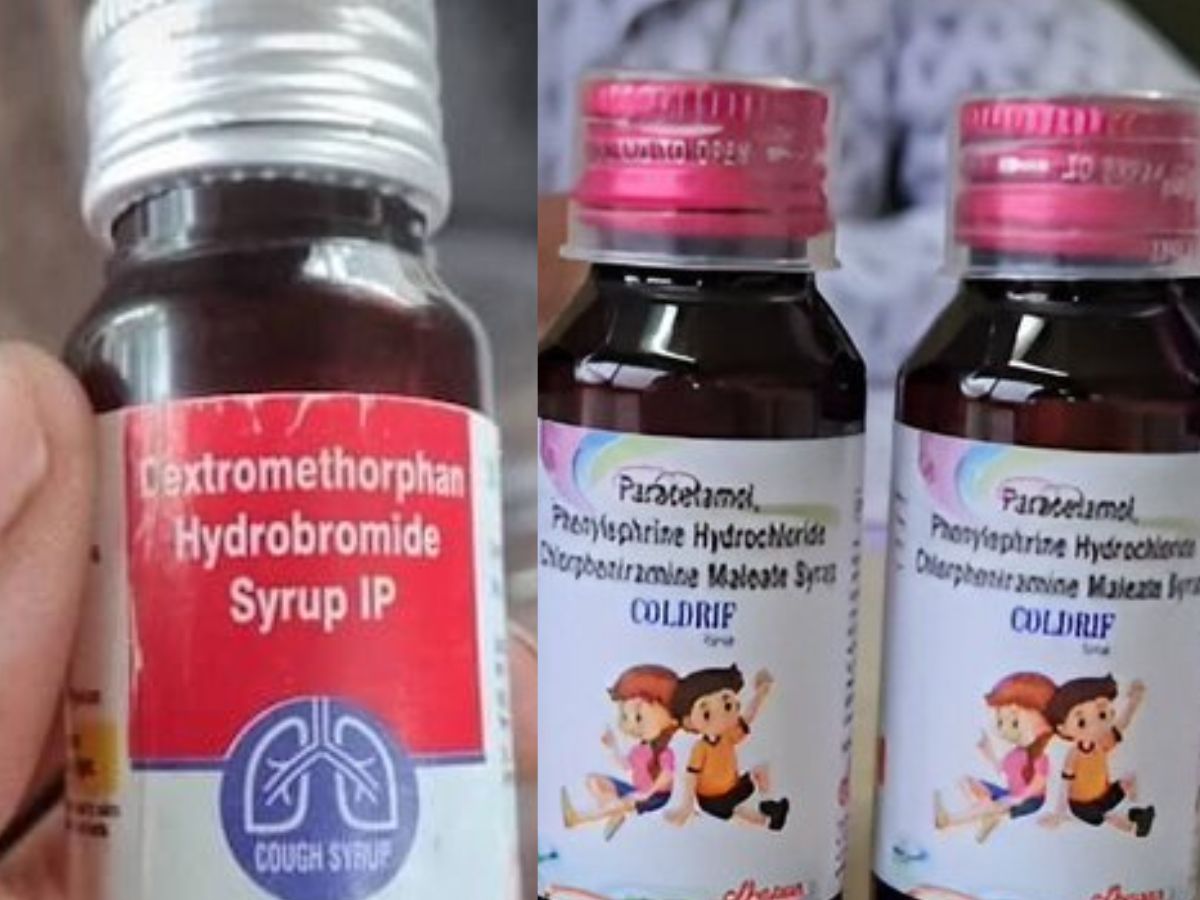
The Tamil Nadu government also banned sales of the syrup immediately after receiving the alert from Madhya Pradesh on October 1. The Tamil Nadu-made Coldrif cough syrup, along with Respifresh TR from Rednex Pharmaceuticals and ReLife from Shape Pharma, has triggered major alarm and banned by TN Government after several children died in Madhya Pradesh. Tests revealed that Coldrif was dangerously contaminated with the toxic chemical diethylene glycol (DEG), with concentrations exceeding 48%, far above the permissible limit of 0.1%.
Tamil Nadu Cancels Sresan Pharma License
On Monday, the government of Tamil Nadu canceled the license of Sresan Pharmaceuticals, which produced the tainted Coldrif syrup associated with the deaths of 22 children in Madhya Pradesh. “The manufacturing license has been canceled completely and the company has been shut down,” a statement from the Tamil Nadu Health Department read.
Arrest of Manufacturer and Officials Suspended
Sresan Pharmaceuticals owner G Ranganathan, from Kancheepuram district, Tamil Nadu, was arrested from his Chennai home last week by a Madhya Pradesh SIT. In the wake of the incident, state drug inspectors in Tamil Nadu were suspended in Kancheepuram for not conducting a check on the pharmaceutical unit since 2022.
Contamination found during investigation
Following Madhya Pradesh’s drug regulator alerting Tamil Nadu on Oct. 1, the state did an independent test. Testing the samples of the same batch validated contamination. The government of Tamil Nadu subsequently informed Madhya Pradesh, the Union Ministry of Health and Family Welfare, and other states where the syrup had been supplied, Odisha and Puducherry.
Officals stated, “The suspected batch was produced with a non-pharmacopoeial grade Propylene Glycol excipient, which could have been adulterated with Diethylene Glycol (DEG) and Ethylene Glycol, which are established nephrotoxic and toxic agents.” On October 3, the samples had 48.6% DEG—486 times the allowed limit.
Immediate Steps to Halt Distribution of Contaminated Cough Syrup
The Tamil Nadu government also banned sales of the syrup immediately after receiving the alert from Madhya Pradesh on October 1 to avoid private players using it. The state also clarified that government hospitals and clinics never purchased Coldrif syrup since all medicines are bought through the Tamil Nadu Medical Services Corporation (TNMSC).
A “stop production” notice was directed to Sresan Pharmaceuticals on October 3. On October 5, the state sent a notice to the company inquiring why their license should not be completely revoked and requesting the same within 10 days. Ranganathan and analytical chemist K. Maheswari received a show-cause notice on October 7.
WHO Flags Coldrif Among Contaminated Syrups
The WHO has identified three specific syrups that have been affected: Coldrif from Sresan Pharmaceuticals, Respifresh TR from Rednex Pharmaceuticals, and ReLife from Shape Pharma. The Central Drugs Standard Control Organization notified the WHO that these syrups were ingested by children under five in Chhindwara in Madhya Pradesh, with the outcome being fatal.