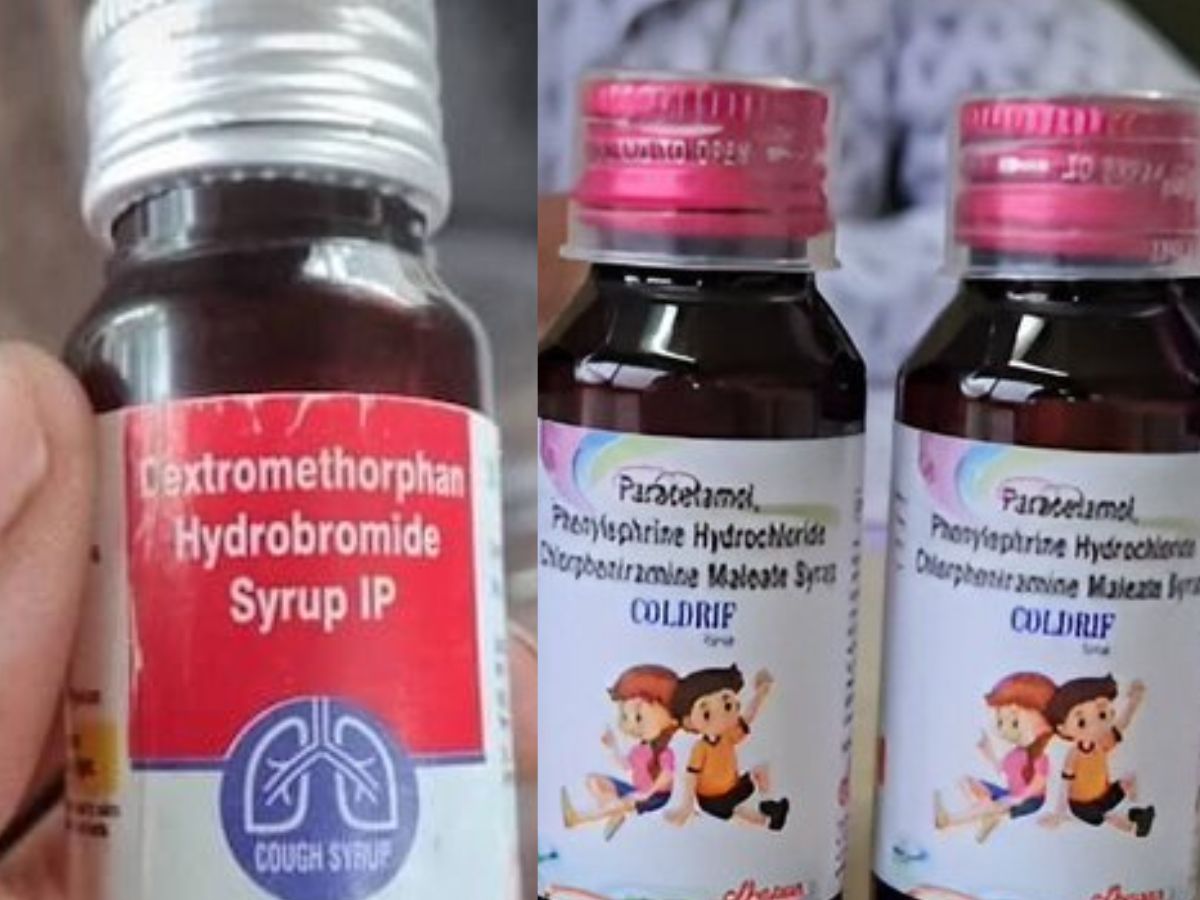
The Kerala government has suspended the sale of Coldrif cough syrup across the state as a precautionary measure. This decision comes after several other states took similar steps in response to safety concerns.
Officials said the move follows a report from the Health Ministry, which confirmed that samples of the syrup tested positive for diethylene glycol (DEG) a toxic solvent used in industrial products. The chemical, found beyond permissible limits, can lead to serious kidney damage or even death.
Why Coldrif Cough Syrup Is Under Scrutiny
Coldrif, marketed by Sresan Pharmaceuticals based in Kanchipuram, Tamil Nadu, has been in the spotlight after at least 14 children died in Madhya Pradesh’s Chhindwara district since September 7. Health officials suspect the deaths were linked to kidney failure caused by the contaminated cough syrup.
According to officials, 10 of the 14 deaths occurred in the Parasia subdivision. The Madhya Pradesh government responded by banning the syrup’s sale last week. Reports also suggested that Rajasthan recorded similar child deaths possibly connected to the same medicine.
Toxic Chemical Found in Syrup Samples
Tests revealed that the Coldrif syrup contained 48.6% diethylene glycol, a highly poisonous substance. The Drug Testing Laboratory in Chennai, under Tamil Nadu’s Directorate of Drug Control, tested the syrup and declared it “Not of Standard Quality.” The findings confirmed that the product violated safety norms, raising alarms across several states.
Multiple States Ban Coldrif Syrup
After Tamil Nadu and Madhya Pradesh, Kerala has now banned the sale of Coldrif cough syrup and ordered its removal from all markets. The ban took effect from October 1.
An official from the Food Safety and Drug Administration Department in Tamil Nadu said, “With effect from October 1, the sale of the cough syrup manufactured by the city-based firm has been prohibited across Tamil Nadu.”
Even though a preliminary probe in Kerala showed that the affected batch had not been distributed in the state, authorities decided to impose a complete suspension as a preventive measure.
Telangana Issues Public Warning
The Telangana Drugs Control Administration (DCA) also issued a “public alert – stop use notice” for Coldrif cough syrup. Officials said they were alerted by reports of child deaths in Madhya Pradesh and Rajasthan.
According to a DCA release quoted by PTI, the public was urged to immediately stop using the syrup and report any health issues linked to it.
CDSCO Launches Nationwide Inspection
The Central Drugs Standard Control Organisation (CDSCO) has launched risk-based inspections of pharmaceutical units across six states Himachal Pradesh, Uttarakhand, Gujarat, Tamil Nadu, Madhya Pradesh, and Maharashtra.
The inspections began after the agency collected 19 drug samples, including cough syrups, antipyretics, and antibiotics, for quality testing. Officials from the Health Ministry said the move aims to identify manufacturing lapses and prevent future tragedies.
Also Read: Centre Holds Emergency Meeting After Children Die From Contaminated Cough Syrup in MP, Rajasthan
Centre Issues Advisory on Cough Syrup Use
The Union Health Ministry issued an advisory to all states on Friday, warning against the use of cough and cold medications for very young children.
The Directorate General of Health Services (DGHS) clarified that “cough syrups are generally not recommended for ages below five years.” For children above that age, doctors should only prescribe them after “careful clinical evaluation with close supervision and strict adherence to appropriate dosing, the shortest effective duration, and avoiding multiple drug combinations.”
The Centre also stressed that cough syrups must not be given to children below two years under any circumstances.
Health Officials on High Alert
With multiple states enforcing bans and inspections, the Health Ministry has prioritized the safety of children. The recent actions aim to ensure that contaminated medicines are removed from circulation and stricter checks are imposed on drug manufacturers. Officials emphasized that protecting public health remains their top priority as investigations into the Coldrif case continue.
Also Read: Who Is Responsible for Cough Syrup Tragedy in Chhindwara And Why The Doctor Arrested?